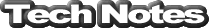 Page 10
Page 10
Three-way catalytic convertor
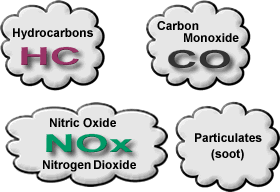
Figure 16 - Major vehicle exhaust pollutants
The next generation of cats dealt directly with the NOx pollutants too, hence the term '3-way' to describe the chemical conversion of three of our exhaust pollutants.
The internal construction is similar to the two-way cat but three precious metals are used as catalysts - a combination of platinum, palladium and rhodium. This mixture of catalysts promotes the reaction of Carbon Monoxide (CO) with Nitric Oxide (NO) to produce Nitrogen (which is harmless) as well as the reactions found in the two-way catalyst, turning HC and CO into CO2 and water.
Figure 17 shows the reactions occurring inside the catalyst. The concepts of Reduction and Oxidation ("redox") are the most fundamental reactions in chemistry, but in this situation will only take place with the catalysts present.
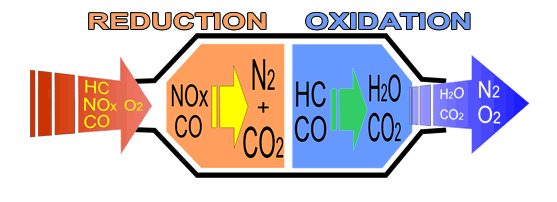
Figure 17 - Internal reactions in the three-way catalyst
It can be seen that the exhaust gases coming in and out of the catalyst have a gas present which we have not explained yet - Oxygen (O2). This creates a problem for our engine management system and is one of the shortcomings of the three-way catalyst.
Stoichiometric combustion has no excess of Oxygen leaving the engine, but in order for the Oxidation reaction to work properly we need an excess of Oxygen. An obvious solution may be to use a Smog pump as seen with the two-way catalyst, but unfortunately this will not work here.
A deficit (lack) of Oxygen will mean that the Hydrocarbons (HC) will not be able to be burnt off. Conversely, an excess of Oxygen means that the Nitrogen Oxides cannot reach the already-oxidised catalyst surface. This means that the two halves of the catalyst each work best under different, conflicting conditions.
It is convenient that the narrowband lambda sensor has the effect of the ECU cycling the richness of the mixture back and forth around stoichiometry (as we have seen earlier) - this means the exhaust gas stream will cycle between an excess of Oxygen then a deficit of Oxygen.
Although this is far from ideal, it simplified the control systems of early catalysts and makes sure that each part of the cat has favourable conditions at least part of the time, but even then only when the system is operating in closed-loop mode.
Later (OBD-II) catalysts have the element Cerium added to the reduction part of the cat - this is an element capable of 'storing' Oxygen and releasing it later - which enables us to combat the problem of when the car is running in open-loop mode. The stored Oxygen will automatically be released under open-loop conditions which keeps both halves of the cat working, even during acceleration or other open loop modes.

