 Page 11
Page 11
New Challenges for Catalytic convertors
Catalytic convertors have massively reduced emissions from vehicle engines and have massively improved the air quality - in fact without them it would be completely impossible to sustain the number of vehicles we have on our roads. Regulations governing emissions are however constantly being tightened and catalyst manufacturers are having to improve the efficiency of catalysts under cold-start conditions, and are having to develop new types of emission controls to deal with diesel exhaust and other lean-burn fuelling strategies.
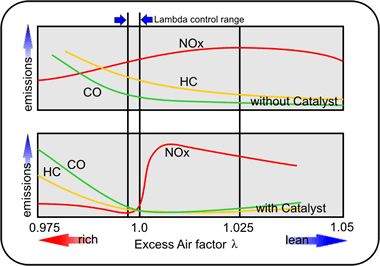
Figure 18 - The effect of the catalyst on Vehicle exhaust Emissions
-
this diagram can help you understand MOT emissions failures
All classes of vehicles now have to meet ultra-low emissions targets. This creates a number of problems. Firstly, most hydrocarbons and carbon monoxide is produced when the engine is starting and warming up, and the catalyst is still cold. One strategy used to combat this is to have the catalyst element 'close-coupled', ie. very close to the engine. Another is to have a small "pre-cat" device that functions like a normal catalyst but gets up to temperature far more quickly, thus reducing the emissions soon after start-up.
An increasing number of engines use these types of cat, but it has taken many years to develop a catalyst substrate that is mechanically stable over a very wide range of temperatures. The reason catalysts are traditionally further down the exhaust stream is that the exhaust gases have had chance to cool a little during their short journey from the engine, and as a result place less stress on the ceramic substrate.
The shortcoming of this arrangement is that the catalyst takes much longer to heat up to operating temperature. When cold, the cat will not be converting our pollution groups as well as it should.
Lean-burn engines
Lean-burn engines also create a challenge. They have an advantage in that they do not produce much Carbon Monoxide because of the high air-to-fuel ratio - the resulting excess of Oxygen in the combustion chamber means that there is enough free oxygen around for CO2 (Carbon Dioxide) to be produced instead of CO (Carbon Monoxide). But this also means that more oxides of Nitrogen are produced. Figure 18 above shows how the NOx emissions increase sharply as we lean out the mixture. A lot of this is to do with the elevated combustion temperatures.
A strategy to combat this problem is to include the metal Barium in the catalyst which will store these oxides of Nitrogen in the form of Nitrates until they can react with Carbon Monoxide. Carbon Monoxide is produced by all engines whilst the mixture is enriched during acceleration.
Diesel engines are by nature lean-burn, so the same problem of NOx emissions exists. A strategy that works for the diesel is to inject tiny amounts of fuel directly into the exhaust stream, which then reacts with NOx to make less harmful compounds. This can also be achieved by increasing the amount of fuel injected into the cylinder so some of it reaches the cat unburned.
Diesel engined vehicles also present a different problem - the amounts of particulates (soot) emitted is far greater than for a petrol engine. Filters can be used and are very effective, but they clog up quickly and regenerating (cleaning) them is difficult. The development of new types of sensor will enable a greater degree of accuracy . One possible way out for this is the development of a new part of the catalytic convertor, which first converts nitric oxide into nitrogen dioxide, which then oxidises soot particles that have been collected in a filter.

