 Page 9
Page 9
A bit about Catalysts

Figure 13 - A catalytic convertor
Advances in engine control systems and vehicle technology have allowed modern engines to reduce the amounts of pollutants they produce, but this on its own is insufficient to meet legal emissions targets. Therefore, the problem is tackled by developing systems to react with and clean up this remaining pollution before it exits the vehicle into the atmosphere. Thus the development of the catalytic convertor.
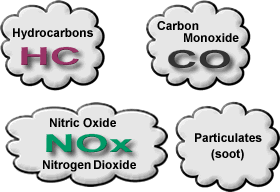
Figure 14 - Major vehicle exhaust pollutants
Most vehicle emission is due to the incomplete burning of fuel. These pollutants include hydrocarbons (HC), Carbon Monoxide, Nitric Oxide and Nitrogen Dioxide, and soot particles.
Nitric Oxide and Nitrogen Dioxide are grouped together and referred to as 'NOx emissions'.
Soot emission is a big problem for diesel engines and a sooty exhaust pipe is a symptom of an over-rich running petrol engine.
One of the major tasks of a correctly functioning sensor is to allow the catalytic convertor to undertake its job of converting environmentally harmful gases into other, less environmentally harmful gases. It can only do this with the precise mixture control of a modern ECU-controlled fuel injection system.
More about Nitrogen
Nitrogen is normally a very unreactive gas. At normal atmospheric pressures, nitrogen and oxygen in the atmosphere will not react, but the very high temperatures and pressures within the engine combustion chamber cause nitrogen to become available for chemical reactions to occur.
The term 'NOx' is used to group together the two main constituents of Nitrogen pollutants from car exhaust - NO (nitric oxide), and NO2 (nitrogen dioxide). NO is produced in the extreme temperatures of engine combustion, and if sufficient oxygen is available, it will then be converted to NO2. NO (nitric oxide) is a colourless, odourless gas. NO2 is a brown coloured gas with a very pungent smell, and irritates the lungs and respiratory airways. It is chiefly responsible for the distinctive smell of non-catalysed exhaust gas on old cars.
NOx pollutants in the atmosphere will react with volatile organic compounds (VOCs - including natural ones such as methane, and artificial ones like petrol vapours, paint thinners, dry cleaning fluid) to form an effect known as 'photochemical smog' by reacting in sunlight. This creates unpleasant irritants such as peroxylacetyl nitrate, and produces low-level ozone, which affects lung function and damages plants and buildings (ozone at a high level protects us from the Suns UV rays, but at ground level it is very harmful).
NOx also reacts with water to produce acid rain. It is obviously important to deal with these dangerous gases, and the catalytic convertor reduces the NOx to harmless Nitrogen (N2) and Oxygen (O).
Nitrous Oxide, N2O, also called laughing gas, is not produced inside the combustion chamber.
Two-way catalytic convertors
Figure 15 - Honeycomb structure maximises surface area. Vehicle catalytic converters use a rounded-off square-shaped chamber, but this is a good approximation to a hexagonal honeycomb and is better for economy of manufacture.
The simplest car exhaust catalytic convertors were developed in the 1960's and were based on a platinum catalyst. This was micro-coated onto a honeycomb alumina support to give maximum surface area. The larger the surface area, the greater the surface available for the chemical reactions to occur.
The convertor dealt directly with only two of our pollution groups (hence the name 'two way') - Carbon Monoxide and unburned Hydrocarbons. They react with oxygen to produce carbon dioxide and water. The NOx emissions are dealt with back in the combustion chamber, where the ECU runs the engine slightly rich all the time to reduce the amount of oxygen available for reaction with Nitrogen.
However, this lack of Oxygen causes another problem. The HC and CO reactions occurring within the two-way cat depend on an abundance of oxygen in the exhaust stream, which of course wasn't present because of the slightly rich mixture, so a device was developed called an 'air pump' (or 'smog pump'). Its purpose was to inject fresh air into the exhaust manifold (ie. after combustion) from an engine-driven pump (not to be confused with an EGR valve). This then gives the catalyst favourable conditions under which to work.
The catalyst will convert our pollutant groups into CO2 and Carbon Dioxide. Carbon Dioxide is naturally present in the atmosphere, and is a reasonably effective 'greenhouse gas'. However, because of the enormous volume of CO2 emitted by the millions of vehicles across the world and other man-made sources it is a cause for concern. It is however considered preferable to CO or oxides of Nitrogen.
Two-way convertors were phased-out in the mid 1980's because of the arrival of the more efficient three-way catalytic convertor. However, the two-way convertor did much to phase out the use of tetraethyl lead additives (which were used to boost the octane rating and to impart lubrication properties to petrol), reducing the amount of environmental lead. As well as being harmful to living organisms, the lead poisons the catalytic convertor and stops it from functioning.
In the United Kingdom, leaded fuel was available right into the mid nineties, although the actual amount of lead in the fuel was diminished greatly leading up to this. Catalytic convertors were only a legal requirement for cars in the UK and the rest of the EU from the 1993 model year onwards although some imported cars could be found with catalysts from the early 1980's onwards.

